

Although Pt1 only received SCGT, Pt2 received TCGT followed by SCGT, and, therefore, we also determined the VCN by tracking the GCsapM-ADA-specific sequence to examine the engraftment of SCGT-derived cells in Pt2 ( Figure 1A). Consistent with previous reports, the exogenous expression of ADA provided a definitive selective advantage to the T cell lineage but not to other lineages. In Pt1, the VCN in T cells was approximately 0.94 per cell, whereas other cell lineages, including B cells, NK cells, monocytes, and granulocytes, showed a VCN of 0.026–0.39 ( Figure 1B). The VCN was calculated using droplet digital PCR (ddPCR) with primers and probe against the packaging signal (Ψ Figure 1A). PB cells were sorted into CD3 + T cells, CD19 + B cells, CD56 + natural killer (NK) cells, CD14 + monocytes, and CD15 + granulocytes, and then genomic DNA was extracted. We calculated the vector copy number (VCN) in the hematopoietic subpopulations to investigate the long-term engraftment of gene-corrected cells. Engraftment of gene-corrected cells in the hematopoietic system Both patients required Ig supplementation to maintain serum IgG levels over 800 mg/dL ( Figure S1).
#Gene therapy for scid skin#
However, he occasionally had mild viral infections, including skin lesions due to verruca vulgaris. Pt2 showed a relatively higher lymphocyte count (300–1,000/μL) with a response to mitogen. Pt1 suffered from mild viral and bacterial infections in the years following the treatment, and her lymphocyte count remained at 200–300/μL.
After more than 10 years, both patients showed partial immune reconstitution. TCGT consisted of repeated infusions of autologous gene-modified T cells with continuous ERT, and SCGT was performed under the same protocol as in Pt1. He received T cell gene therapy (TCGT) with LASN retroviral vectors at 4.5 years old, and insufficient immune reconstitution resulted in the necessity for SCGT at 13.0 years old. The second patient (Pt2) was a male and showed delayed onset as he was affected with severe pneumonia 8 months after birth and started PEG-ADA at 1.5 years old. PEG-ADA was withdrawn, and no cytoreductive therapy was administered before SCGT. SCGT using GCsapM-ADA retroviral vectors was performed at the age of 4.7 years. Graphical abstractīriefly, the first patient (Pt1) was a female and developed clinical symptoms 15 days after birth, and ERT using polyethylene glycol-modified ADA (PEG-ADA) was commenced. The impaired BM microenvironment due to metabolic abnormalities may create space for the engraftment of vector-marked cells in ADA-SCID, despite the lack of preconditioning.
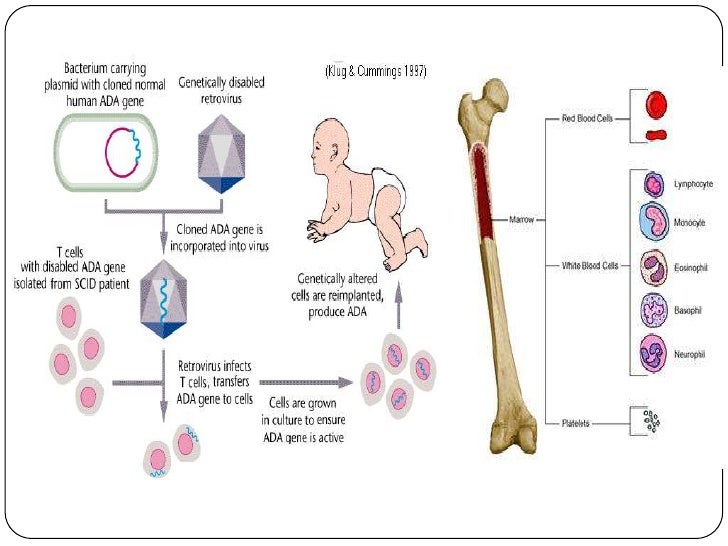
Vector integration sites common to all hematopoietic lineages suggested the engraftment of gene-marked progenitors in Pt1, who showed severe osteoblast (OB) insufficiency compared to Pt2, which might cause a reduction in the stem/progenitor cells in the bone marrow (BM). The use of retroviral vectors yielded clonal dominance of vector-marked clones, irrespective of the lack of leukemic changes. Although neither patient developed leukemia, clonal expansion of SCGT-derived clones was observed in both patients. The distribution of vector-marked cells reflected variable levels of ADA requirements in hematopoietic subpopulations. Moreover, the VCN increased with differentiation toward memory T and B cells. More than 10 years after SCGT, T cells showed a higher vector copy number (VCN) than other lineages. The first patient (Pt1) was treated at 4.7 years old, and the second patient (Pt2), who had previously received T cell gene therapy (TCGT), was treated at 13 years old. Two patients with adenosine deaminase (ADA)-deficient severe combined immunodeficiency (ADA-SCID) received stem cell-based gene therapy (SCGT) using GCsapM-ADA retroviral vectors without preconditioning in 20. Gene Editing: Technology & Applications.


 0 kommentar(er)
0 kommentar(er)
